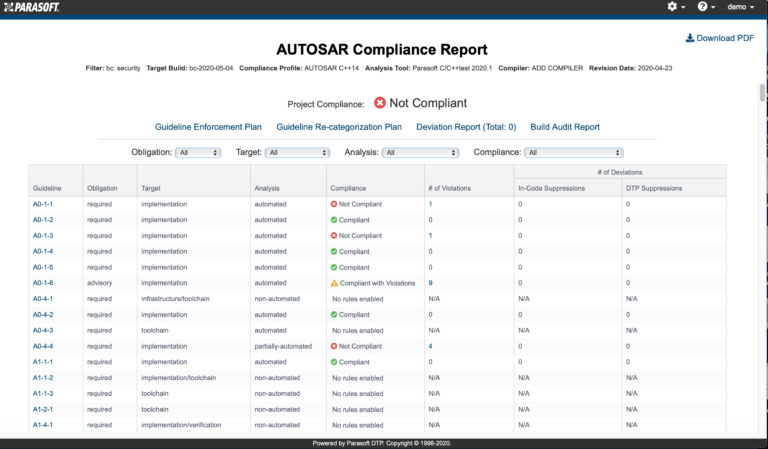
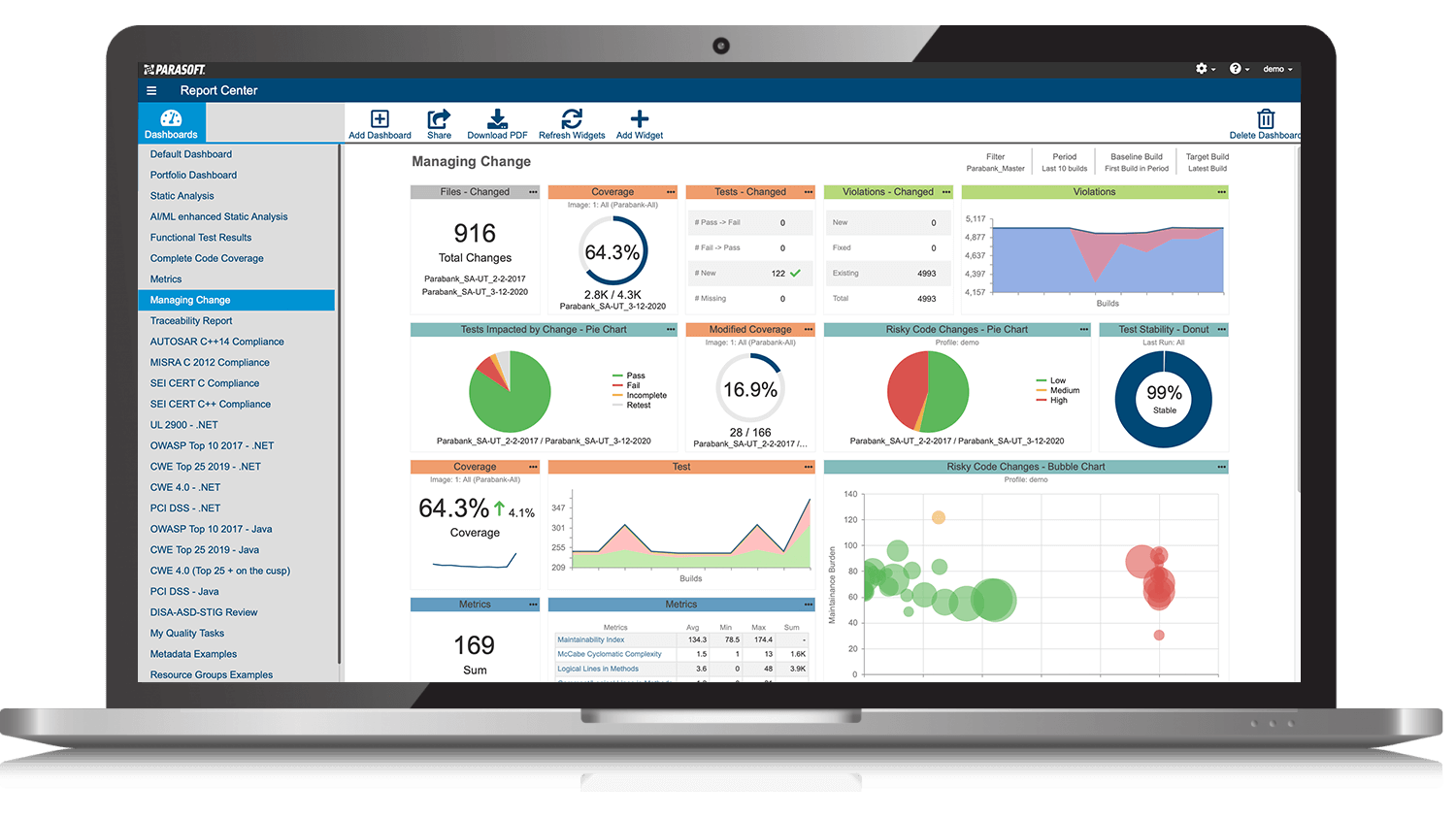
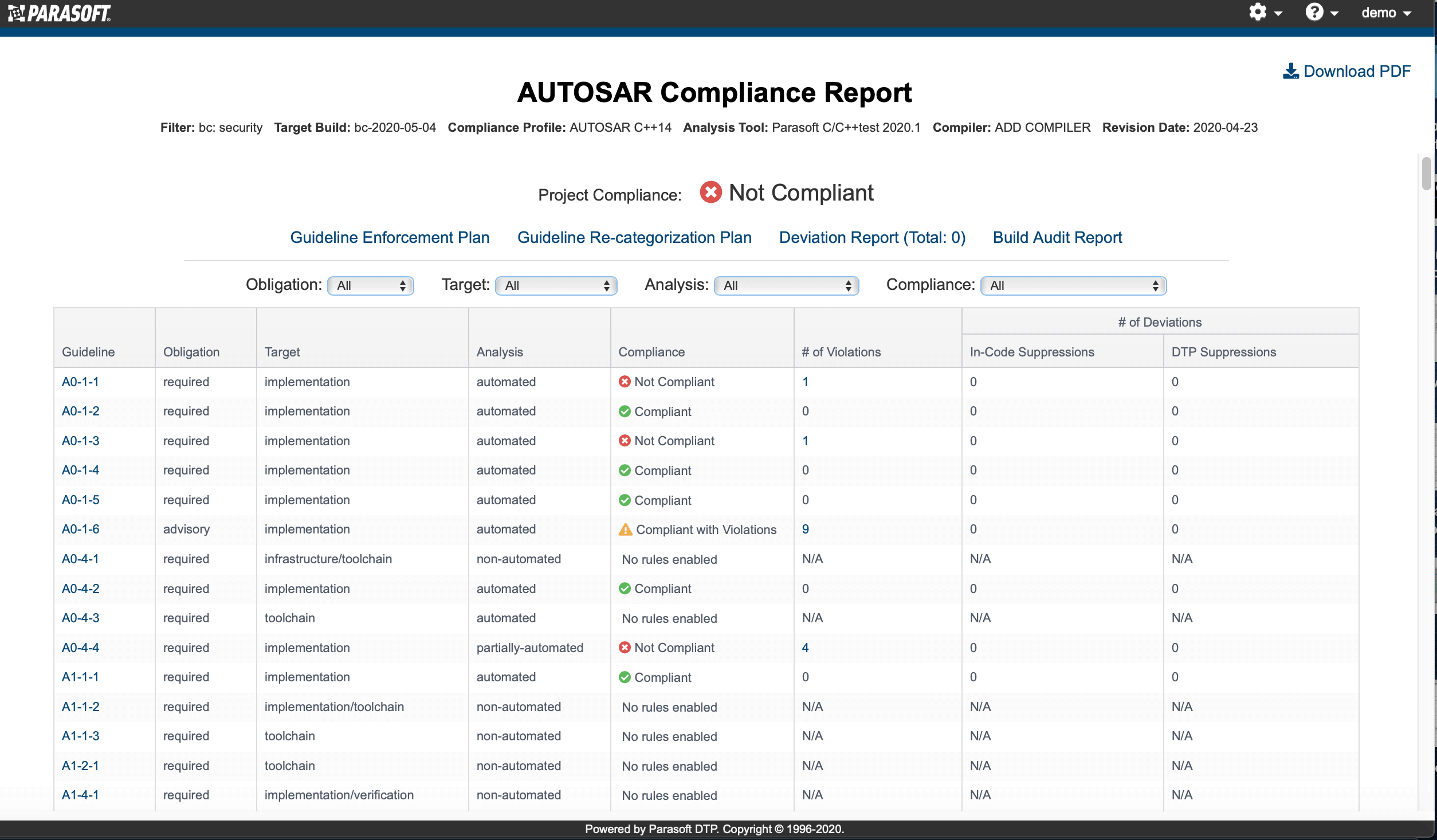
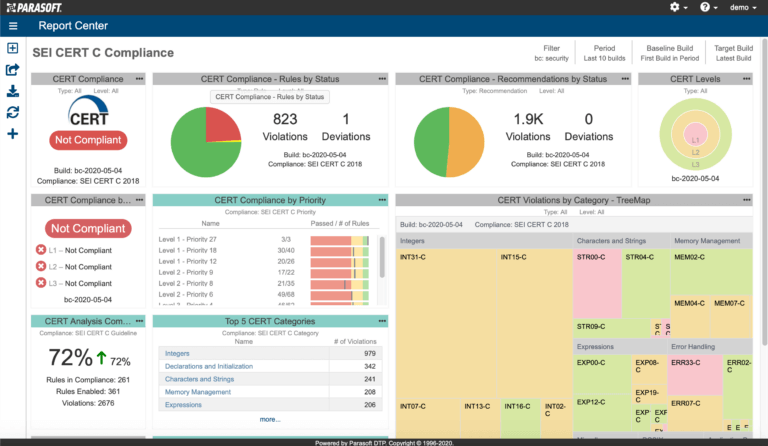
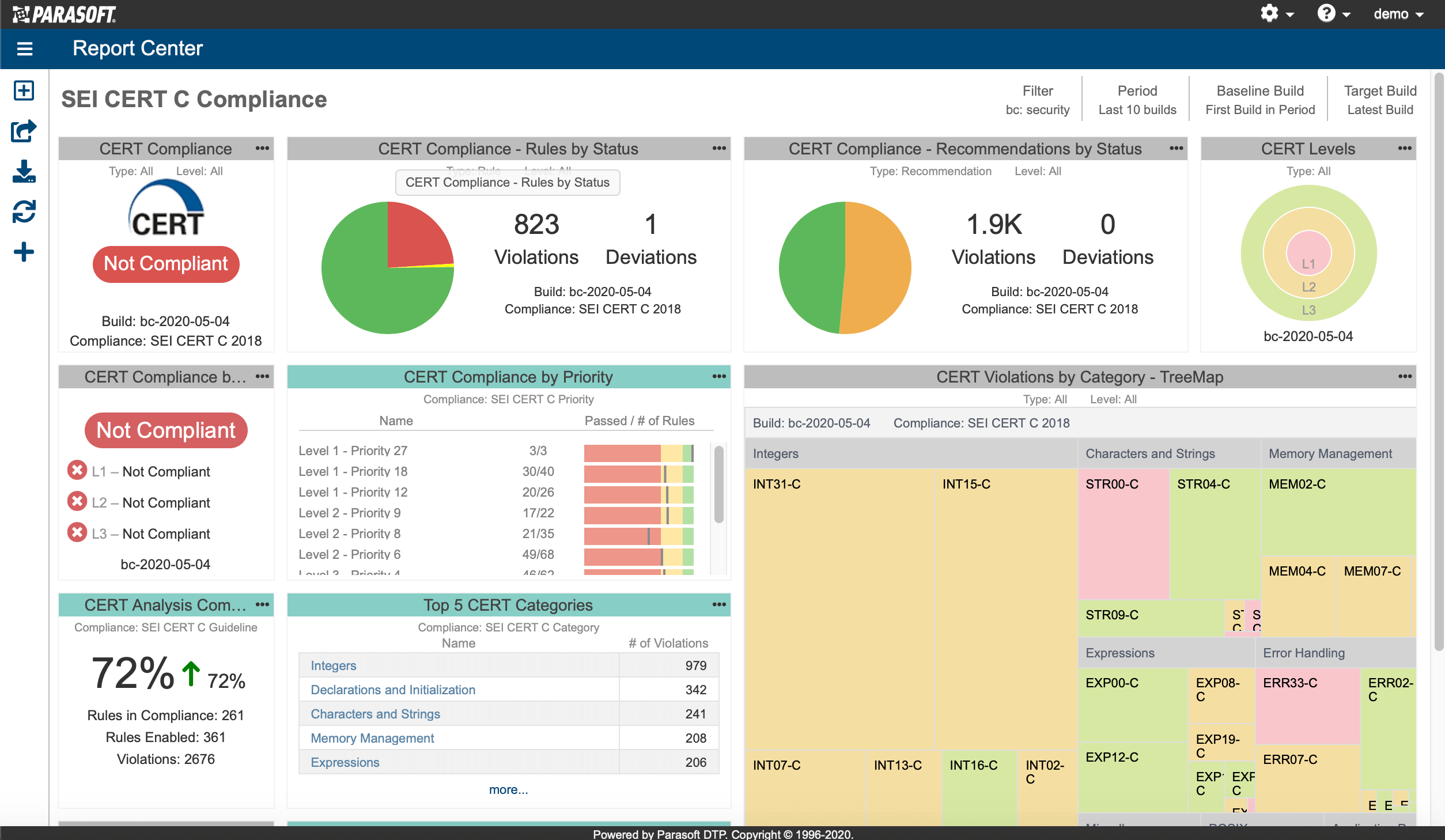
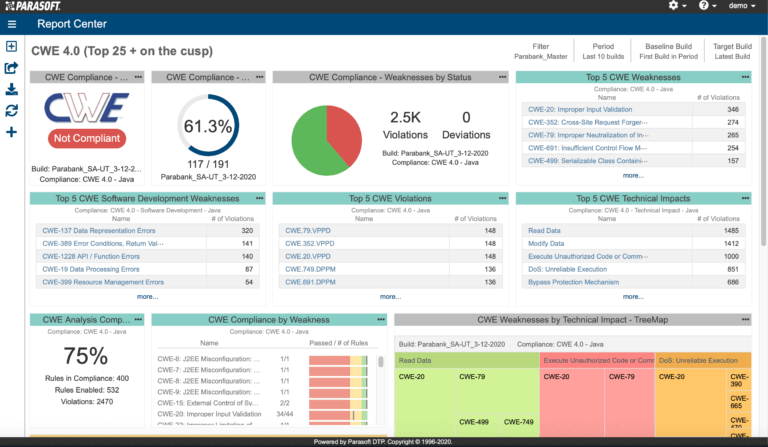
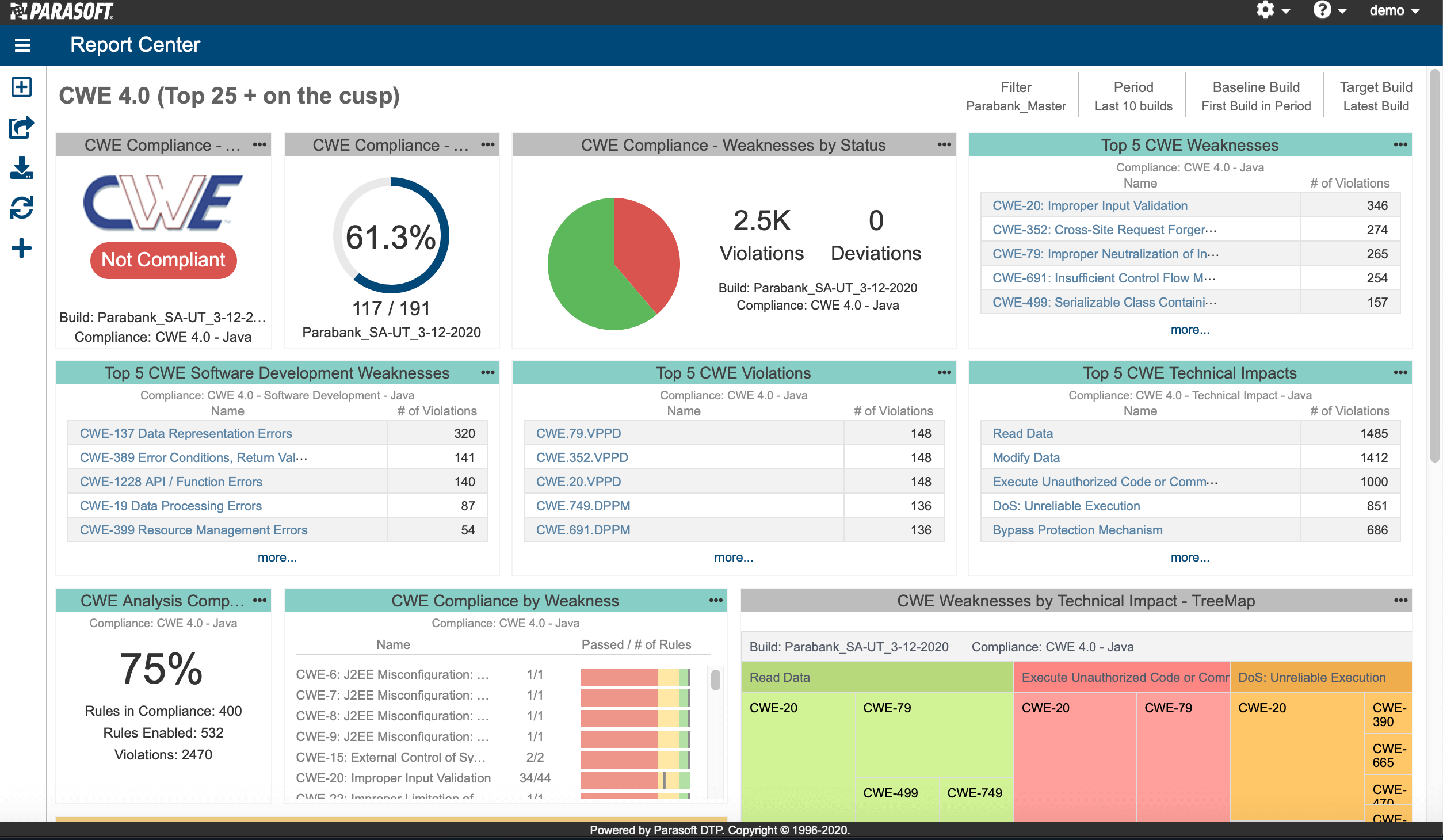
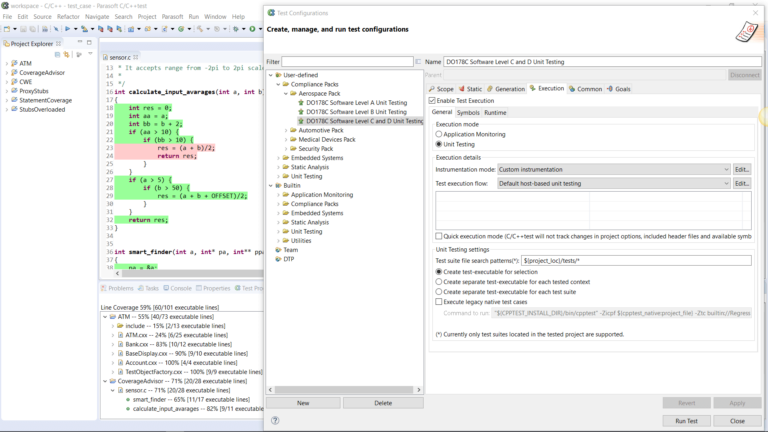
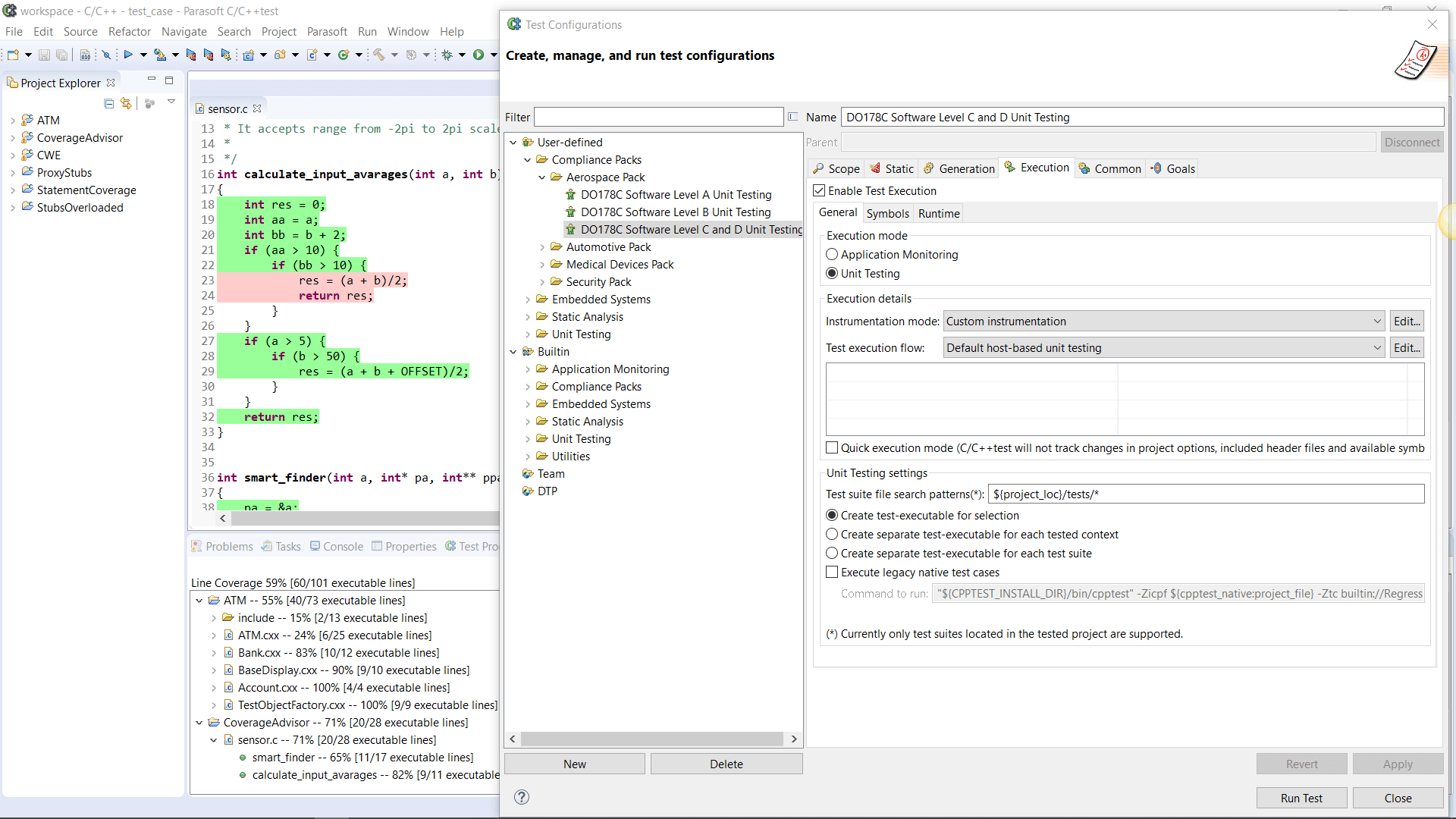
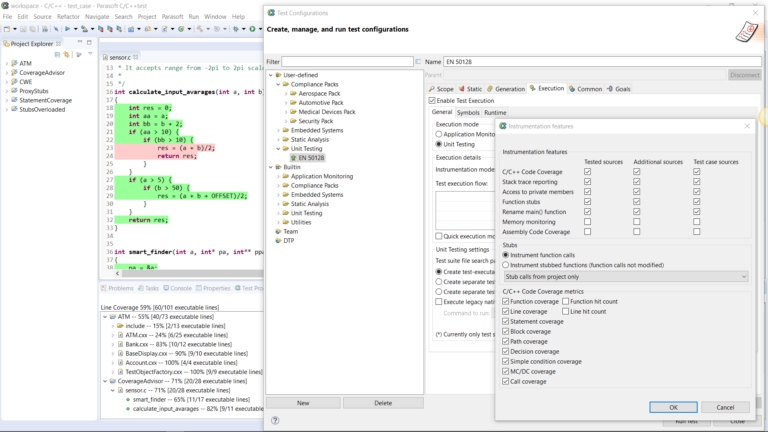
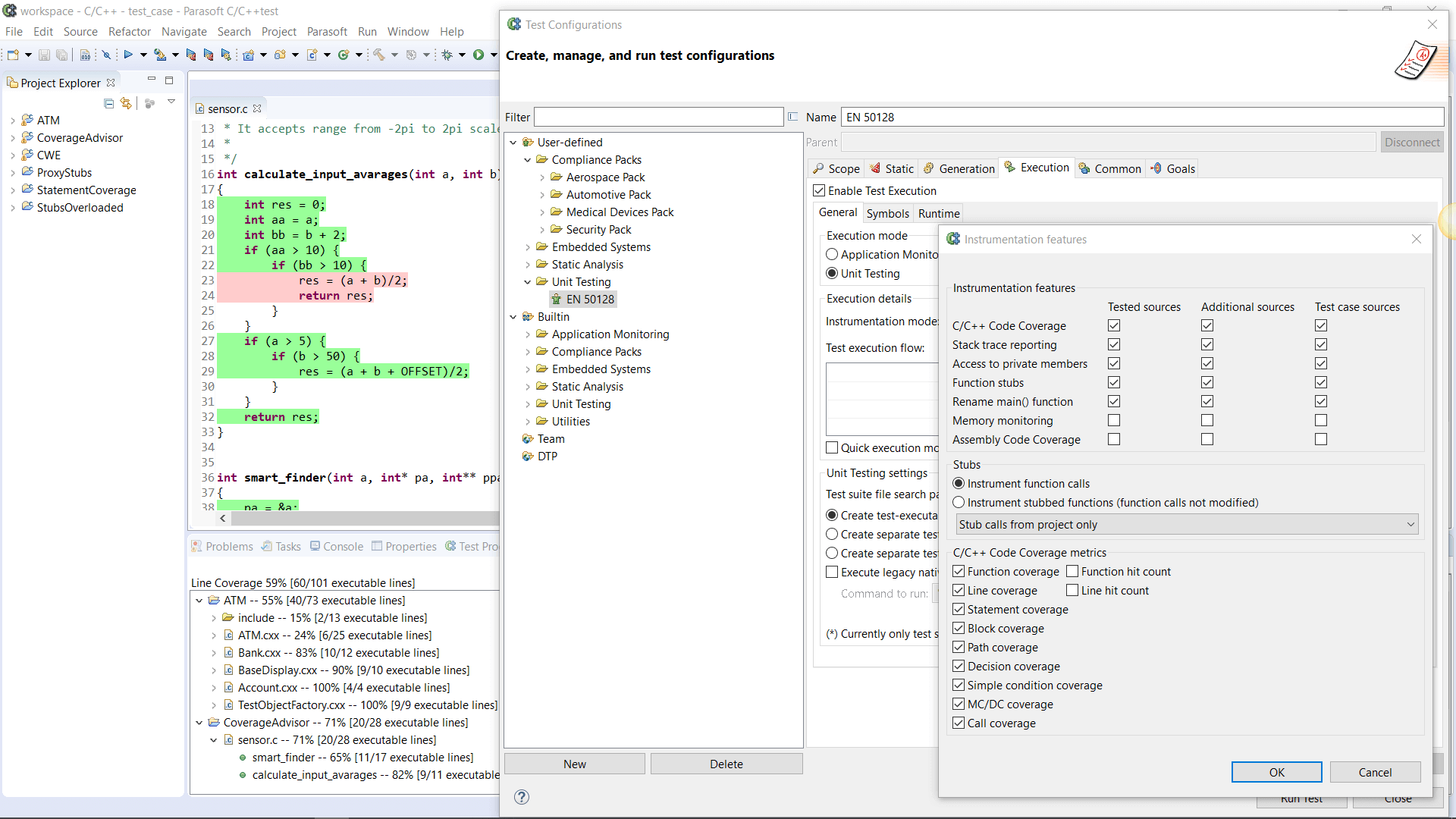
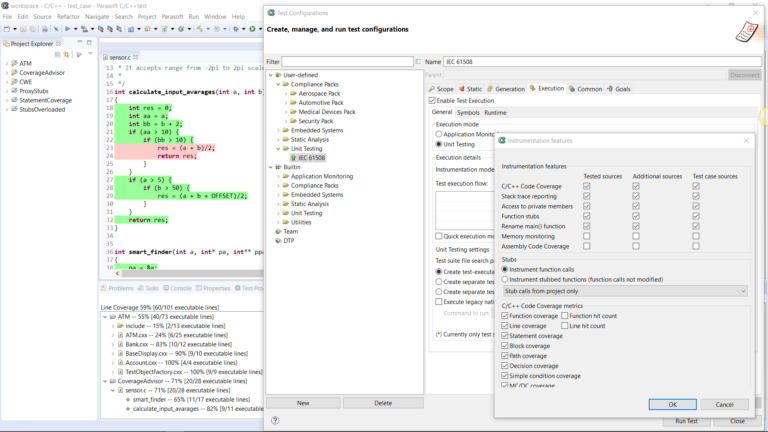
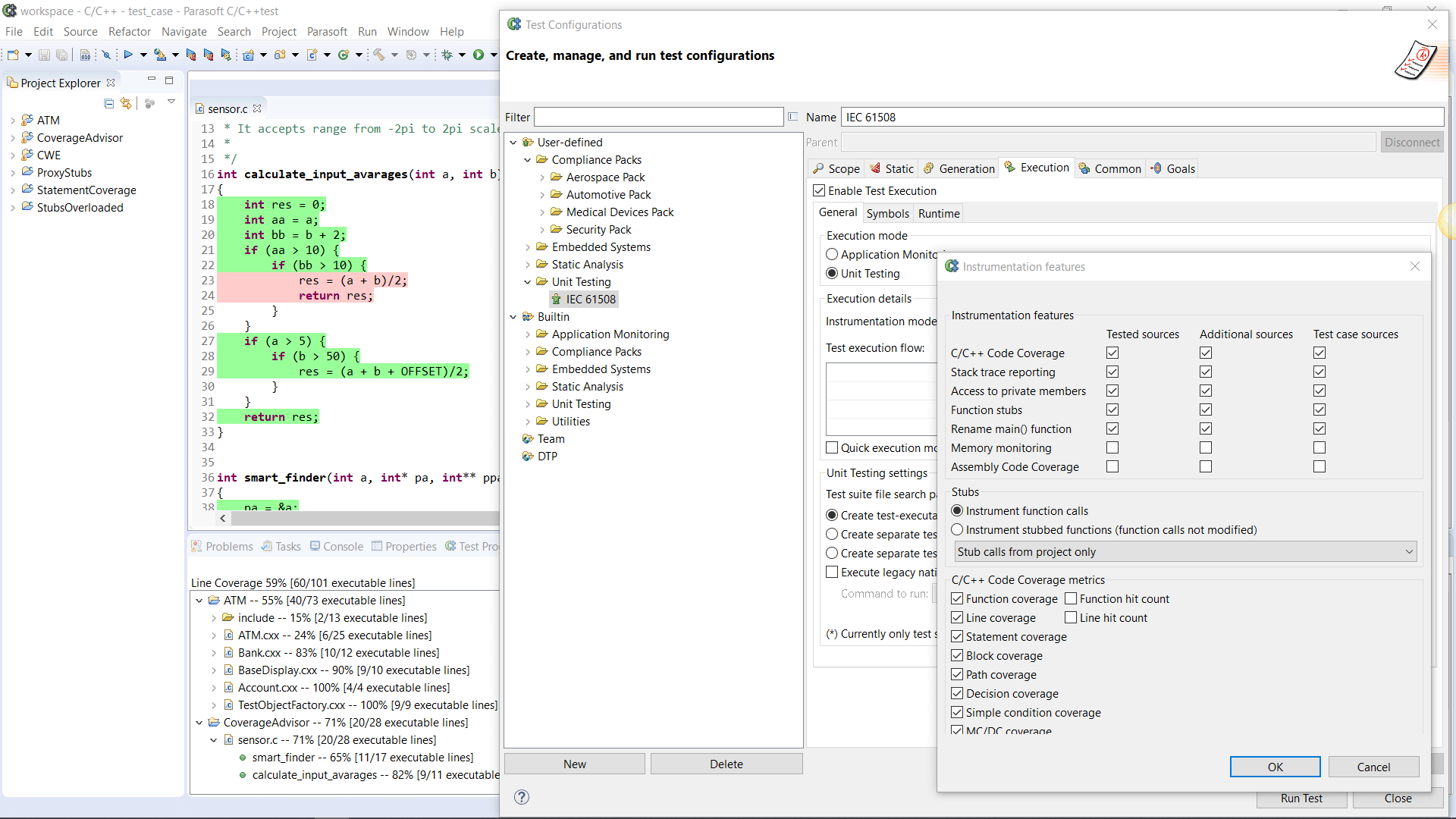
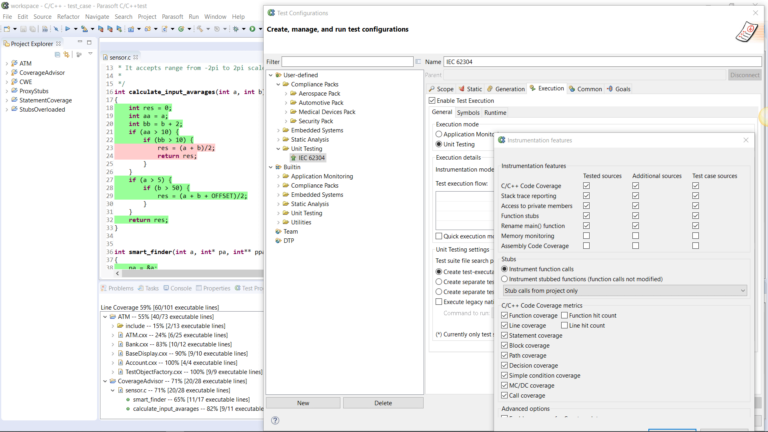
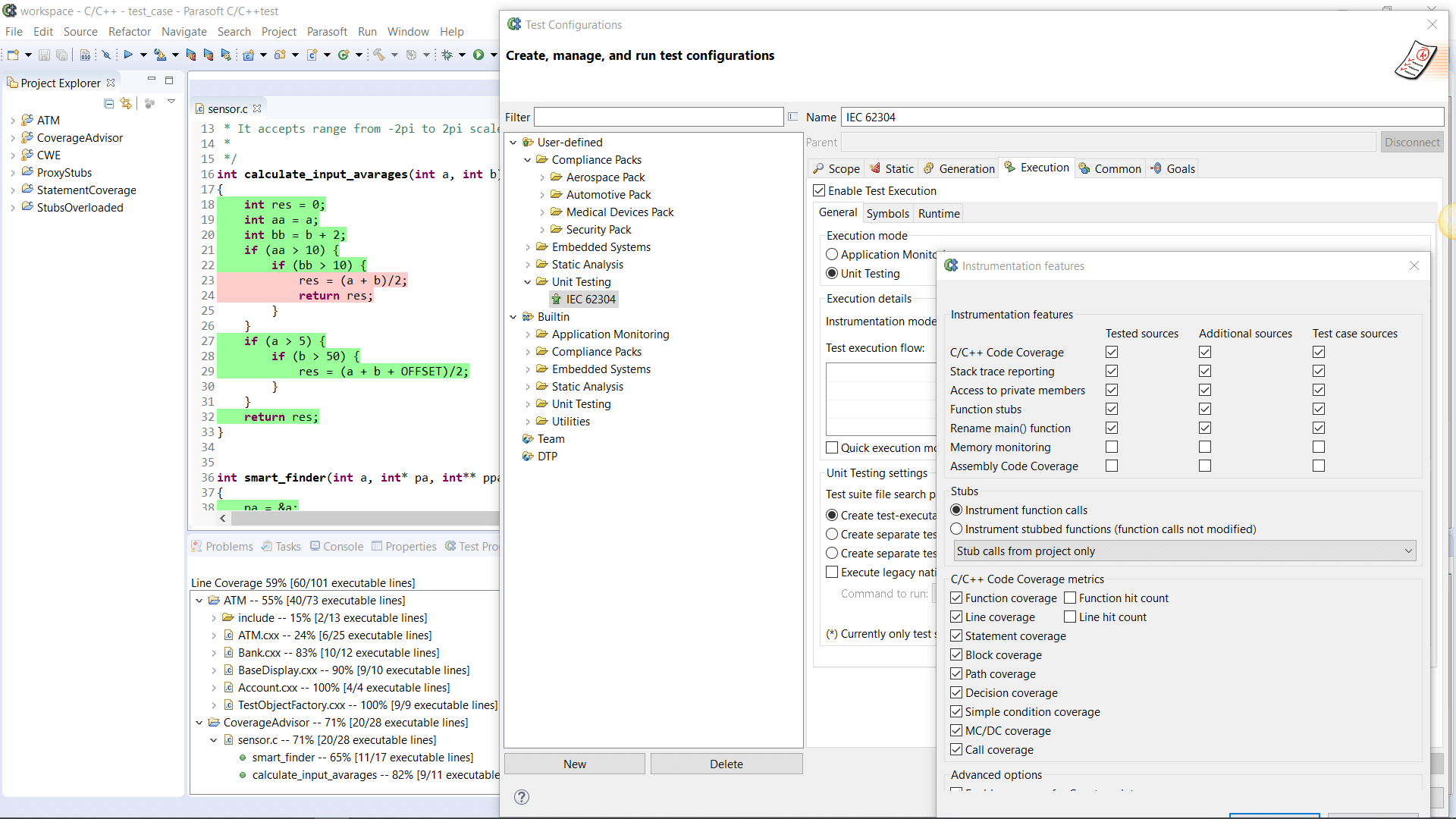
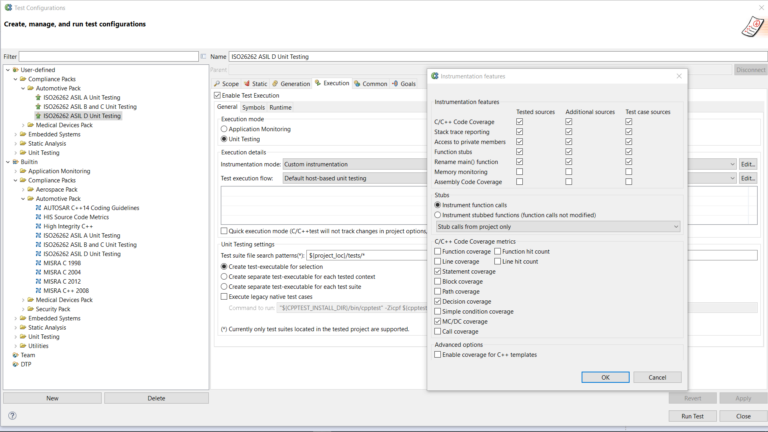
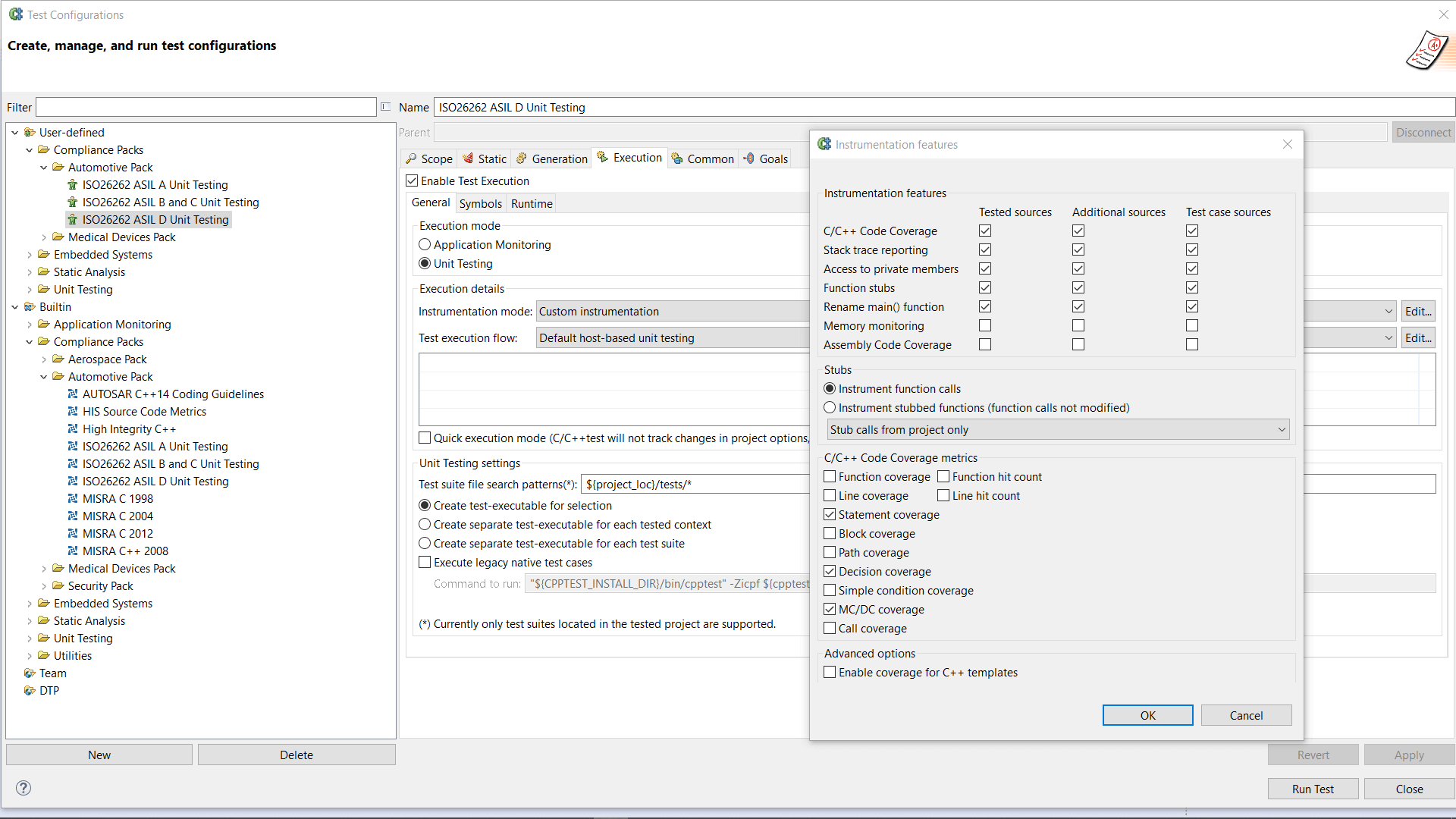
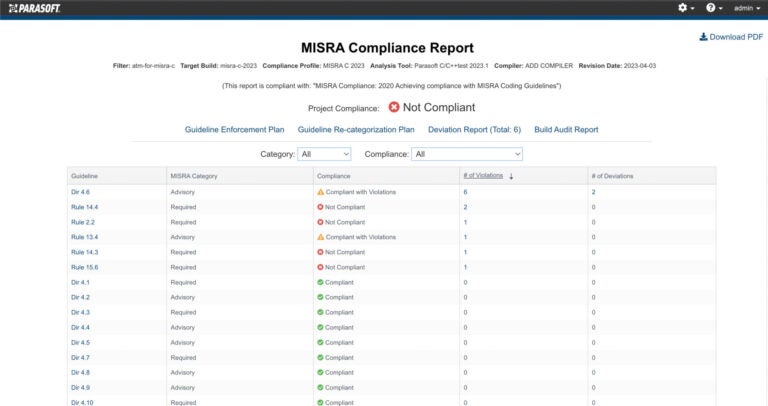
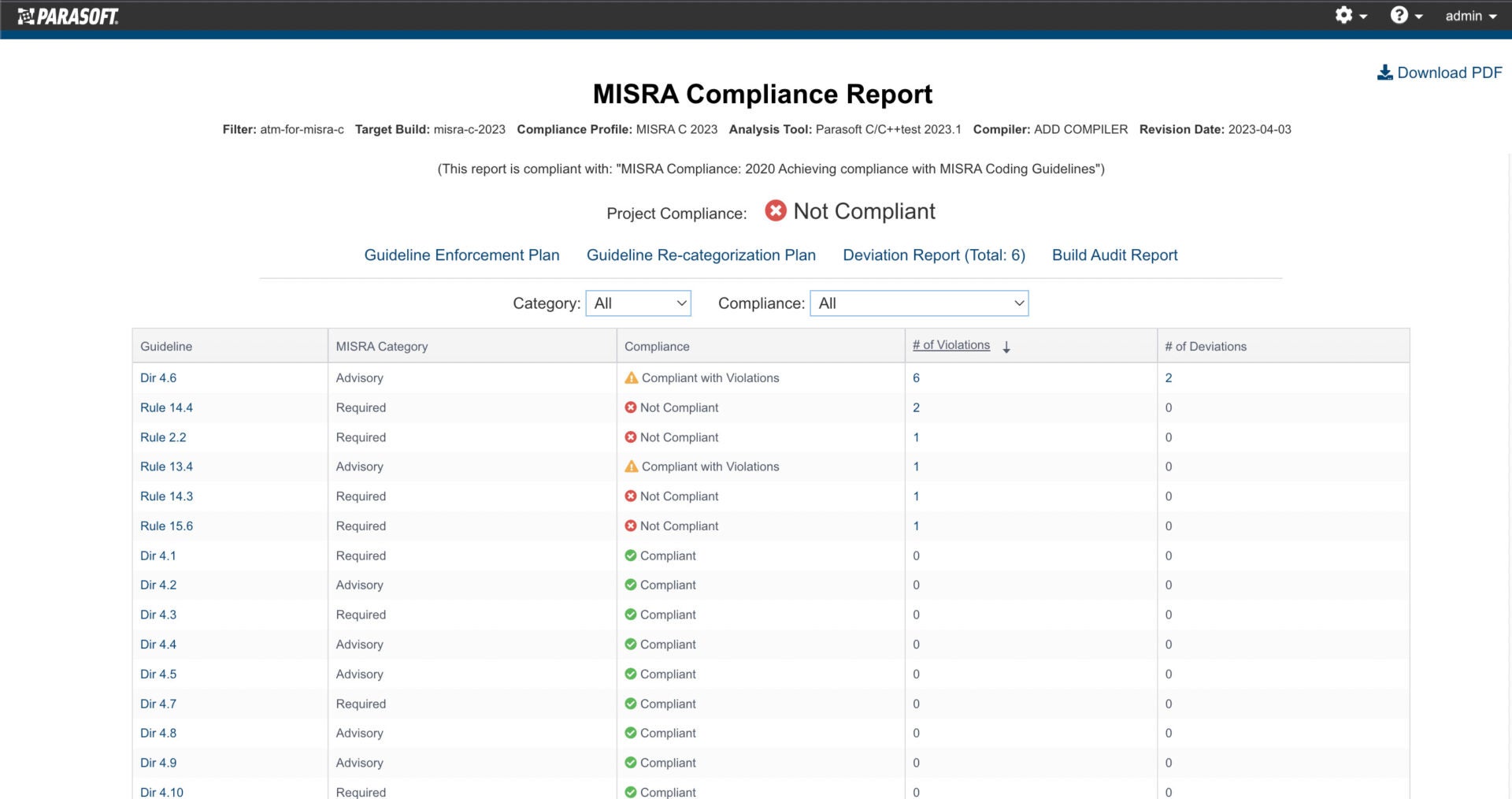
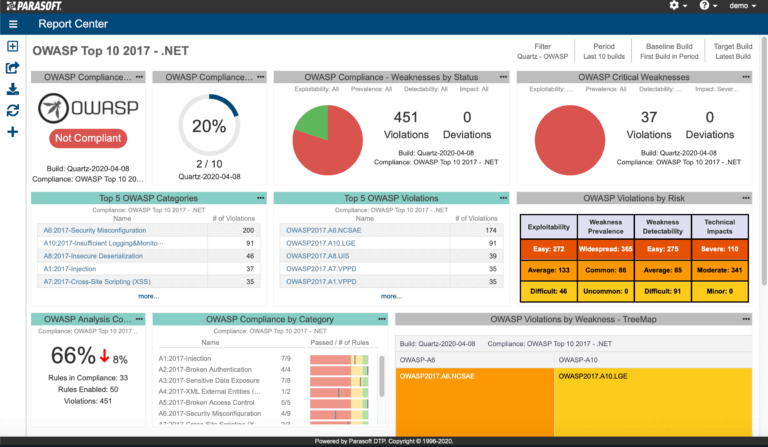
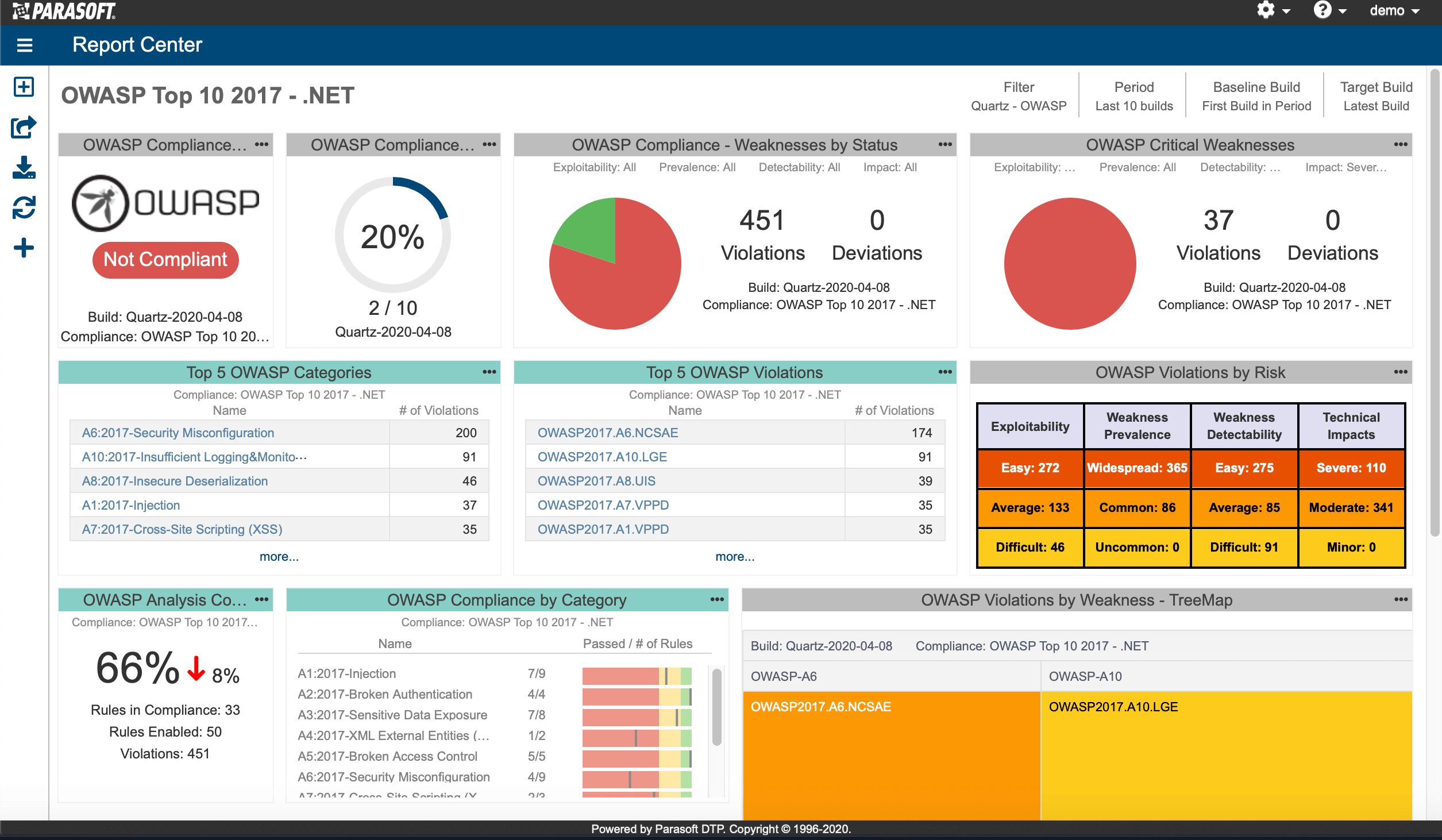
AUTomotive Open System ARchitecture is a worldwide development partnership of vehicle manufacturers, suppliers, service providers, and companies from the automotive electronics, semiconductor, and software industry. Parasoft DTP demonstrates compliance for the AUTOSAR C++14 coding standard.